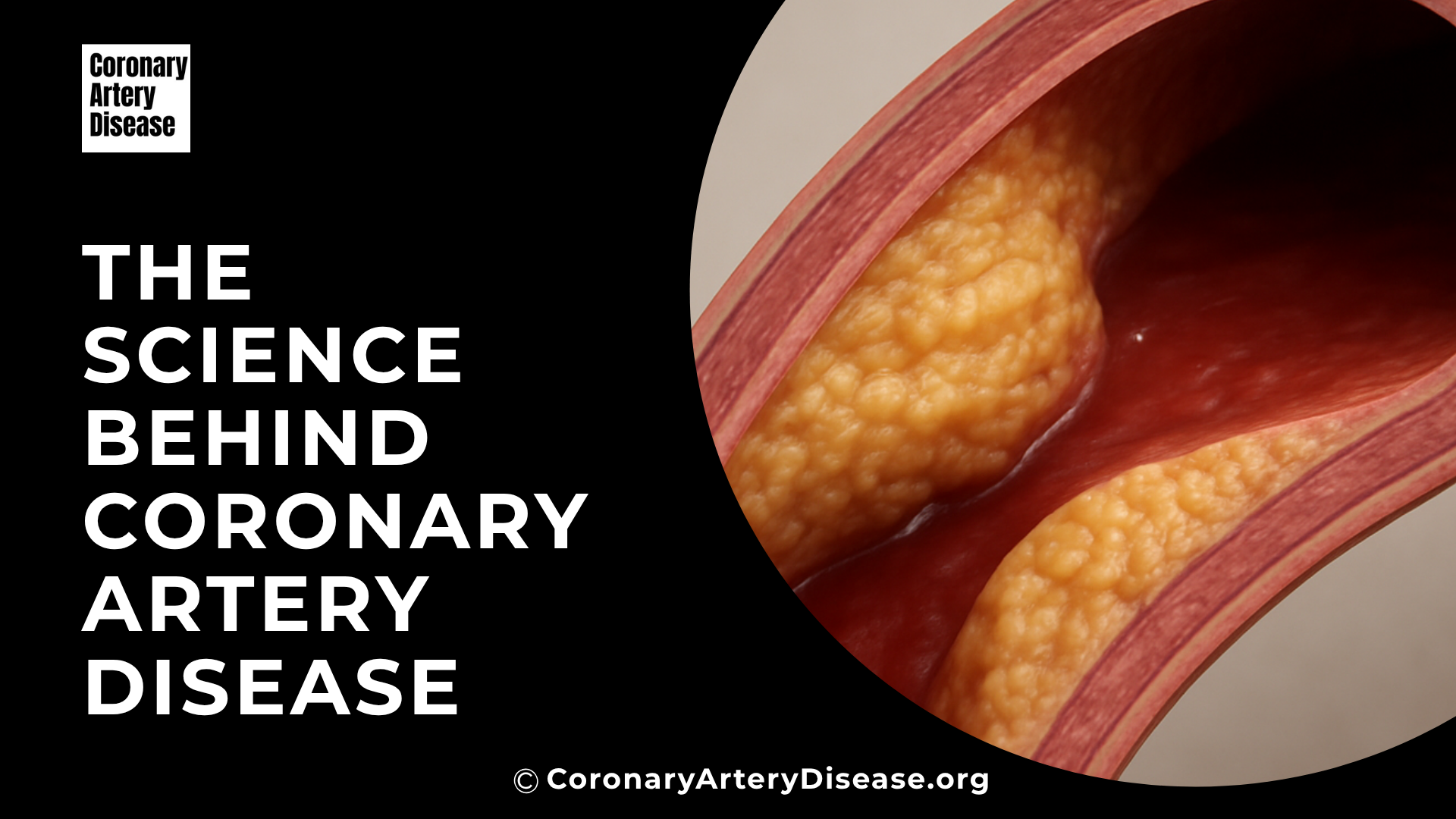
Select your start point from the below categories, and Learn more about coronary artery disease.
Researchers
at Coronary Artery Disease Foundation
Abdelmonim Mohamed Himmett
My story with coronary artery disease began long ago, as I watched my father press his hands tightly against his chest from time to time, so hard that his fingers seemed to dig into his ribs.
Then one morning, I woke to find him collapsed on the floor, gasping for breath in broken intervals. I rushed to apply what we had learned in medical school, performing chest compressions again and again, only to realize later that nothing can outrun death.
Coronary artery disease is the leading cause of sudden death worldwide. I believe we can better understand it, and better treat the disease progression, and complications with advanced research.

For researchers & medical students
This site is structured in Categories, that contain multiple main topics. Once you start reading about a specific topic, you find links that lead to sub topics
I’m human, and I make mistakes.. if you saw one, please comment it, or email it to me

Abdelmonim Himmett
himmett@coronaryarterydisease.org