Overview
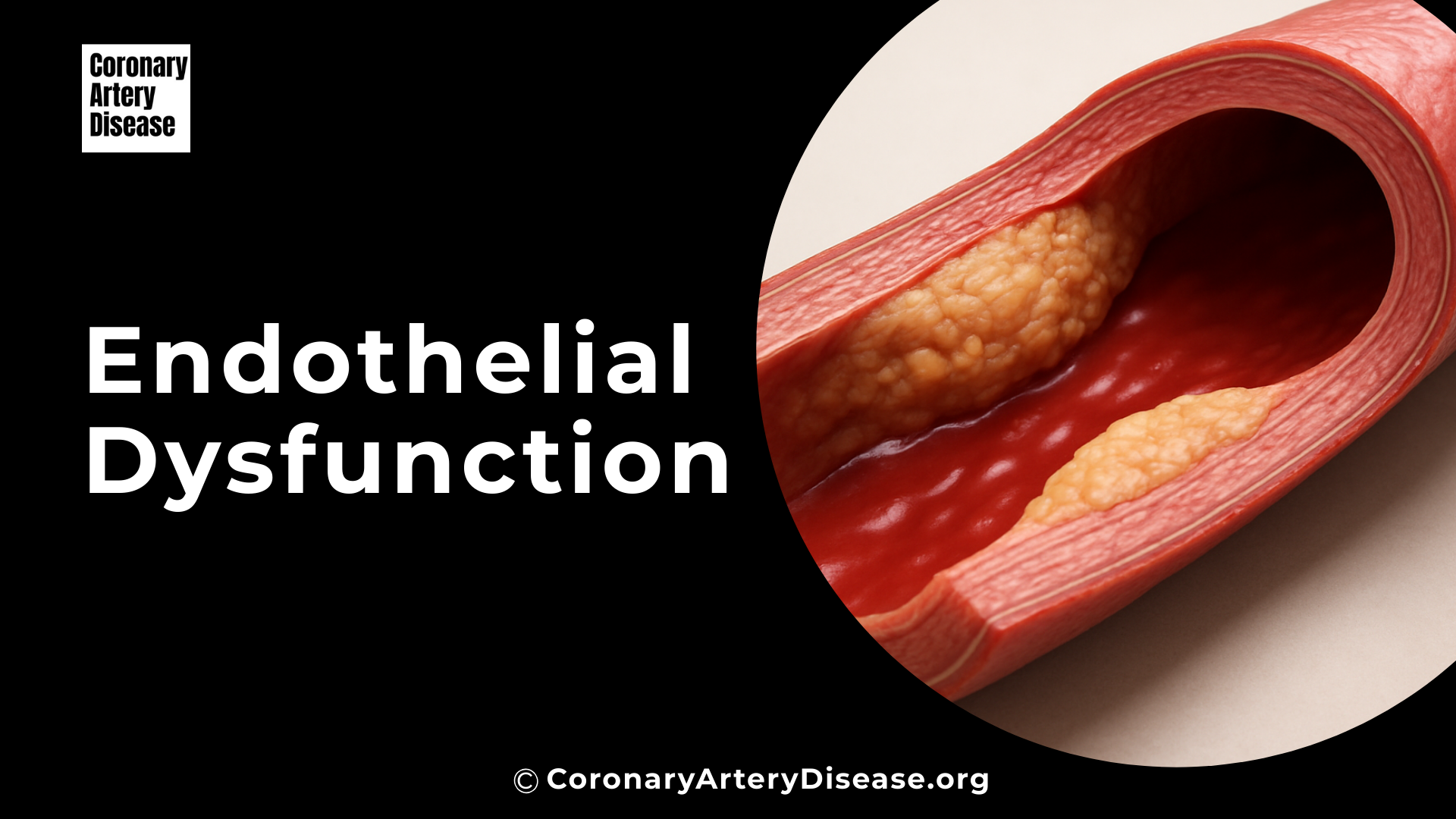
Your blood vessels are lined by a crucial, dynamic layer of cells called the endothelium. This lining is like a smooth, protective shield that helps keep your blood flowing properly and your arteries healthy in a process known as vascular homeostasis. However, when this lining gets damaged or doesn’t work as it should, it’s called endothelial cell dysfunction (ECD). ECD is a significant contributor to serious conditions like atherosclerosis, which is the hardening and narrowing of arteries, and its complications such as heart attacks and strokes.
Understanding what triggers endothelial cell dysfunction is important because it’s the earliest detectable change in the development of atherosclerosis and can even predict future heart-related problems. Many factors, from your lifestyle to environmental exposures and underlying health conditions, can lead to endothelial dysfunction, essentially tipping the delicate balance of your blood vessel health towards disease.
In Details ; What Exactly Triggers Endothelial Dysfunction ?
One of the earliest and most significant signs of this damage is when endothelial cells start to display adhesion molecules on their surface.
Think of these adhesion molecules as tiny sticky tags that pop up on the surface of your blood vessel lining. Their appearance is a direct signal of underlying damage or stress to the endothelium, effectively changing the smooth, non stick surface into one that attracts circulating cells and particles. This stickiness is a key first step in the development of serious conditions like atherosclerosis, because it allows inflammatory cells and harmful cholesterol particles to attach and begin building up plaques.
A quick list of what makes Endothelial cells show their adhesion molecules
- Pro-inflammatory Cytokines
- Oxidized Lipoproteins (e.g., Oxidized LDL)
- Advanced Glycation End Products (AGEs)
- Disturbed Blood Flow (Hemodynamic Forces)
- Bacterial Products (e.g., Endotoxins)
- Certain Viruses
- Chronic Inflammation
- Oxidative Stress
Inflammation and inflammatory cytokines :
One of the primary triggers for endothelial cells to display adhesion molecules is inflammation. When your body experiences an injury, infection, or chronic stress, certain signaling molecules called pro-inflammatory cytokines (such as Interleukin-1 [IL-1], Tumor Necrosis Factor [TNF], and Interferon-gamma) are released.
These cytokines act directly on endothelial cells, causing them to become activated. This activation leads to a coordinated program of gene changes within the endothelial cells, turning on the production and display of endothelial-leukocyte adhesion molecules like Vascular Cell Adhesion Molecule-1 (VCAM-1) and Endothelial-Leukocyte Adhesion Molecule-1 (E-selectin), as well as Intercellular Cell Adhesion Molecule-1 (ICAM-1). These molecules act like sticky anchors on the vessel surface, marking it as a site of potential trouble and initiating the recruitment of immune cells.
Lipoproteins and Advanced Glycation End Products :
Another major set of triggers comes from the chemical environment of your blood. High cholesterol, particularly elevated levels of bad Low Density Lipoprotein (LDL) cholesterol, plays a significant role. When LDL cholesterol becomes chemically altered, or oxidized , it becomes highly damaging to endothelial cells.
These oxidized lipoproteins directly stimulate the endothelium to express adhesion molecules like VCAM-1, initiating the process of plaque formation.
Similarly, in conditions like diabetes with consistently high blood sugar, harmful compounds called Advanced Glycation End Products (AGEs) accumulate in the vessel walls. These AGEs also trigger endothelial cells to switch on the production of adhesion molecules, contributing to the stickiness and furthering the damage.
Disturbed Blood Flow (Hemodynamic Forces):
The physical forces of blood flowing through your arteries, known as hemodynamic forces , are also critical triggers. Atherosclerotic plaques don’t form randomly; they tend to appear in specific areas, such as arterial branch points and curves.
In these regions, blood flow can be disturbed, meaning it’s turbulent or oscillatory, rather than smooth and laminar (undisturbed). This mechanical stress directly causes the endothelial cells to increase the expression of inflammatory adhesion molecules like P-selectin, VCAM-1, and ICAM-1, making these areas more susceptible to immune cell attachment and plaque development.
The Central Role of NFκB :
Many of these diverse triggers—whether they are pro-inflammatory cytokines, oxidized lipoproteins, AGEs, or disturbed blood flow—converge on a common internal master switch molecule within the endothelial cells called Nuclear factor-kappa-B (NFκB). NFκB is a pleiotropic transcription factor, meaning it controls the expression of many different genes. When activated by these harmful stimuli, NFκB moves into the cell’s nucleus and instructs the cell to produce more of these adhesion molecules (like VCAM-1 and MCP-1), as well as other pro inflammatory and pro thrombotic substances.
This makes NFκB a central orchestrator of the endothelial cell’s shift to a damaged, pro-atherosclerotic state, prominently signalled by the increased display of adhesion molecules.
The Consequence :
Once these adhesion molecules are expressed on the endothelial surface, they act as specific binding sites for circulating immune cells, primarily monocytes. These immune cells adhere to the sticky endothelial lining and then migrate into the vessel wall (the sub-endothelial space).
Monocytes can transform into macrophages, which then engulf modified lipoproteins (LDL) and become foam cells – a hallmark of early atherosclerotic lesions. This entire process highlights how the initial appearance of adhesion molecules is a critical, early event in the complex and progressive development of atherosclerosis.
Other Similar Questions
Can doctors measure these adhesion molecules to check for vessel damage?
Yes, increased levels of soluble forms of these adhesion molecules, such as sVCAM-1 and sE-selectin, can be detected in the circulating blood and may serve as markers of endothelial damage and disease severity.
Resources
- Gimbrone, M. A., Jr., & García-Cardeña, G. (2016). Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circulation Research, 118(4), 620–636. doi:10.1161/CIRCRESAHA.115.306301
- Poredos, P., Poredos, A. V., & Gregoric, I. (2021). Endothelial Dysfunction and Its Clinical Implications. Angiology, 72(7), 604–615. doi:10.1177/0003319720987752
- Mannarino, E., & Pirro, M. (2008). Endothelial Injury and Repair: A Novel Theory for Atherosclerosis. Angiology, 59(Suppl 2), 69S–72S. doi:10.1177/0003319708320761
- Wang, X., & He, B. (2024). Endothelial dysfunction: molecular mechanisms and clinical implications. MedComm, 5(e651). doi:10.1002/mco2.651
- Shimokawa, H. (1999). Primary Endothelial Dysfunction: Atherosclerosis. Journal of Molecular and Cellular Cardiology, 31(1), 23–37. Article No. jmcc.1998.0841


Leave a Reply